
Is your practice vulnerable to payment processing fraud and chargebacks?
Todd Shryock is managing editor of Medical Economics.

Is your practice vulnerable to payment processing fraud and chargebacks?

The outlook for medical device manufacturing in 2026

What medical practices need to know about risk in 2026
Results from randomized trial show significant, sustained improvements in pain, function and quality of life for patients with chronic intractable neuropathic pain

Is your practice vulnerable to payment processing fraud and chargebacks?

The outlook for medical device manufacturing in the coming year

What medical practices need to know about risk in 2026

The Agilent S540MD system expands the company’s digital pathology portfolio, aiming to help clinical laboratories improve efficiency, manage growing workloads and integrate AI-driven workflows.

A conversation with Peter Reilly, North American Healthcare Practice Leader, HUB International

What medical practices need to know about risk in 2026

The outlook for medical device manufacturing in the coming year

Whether you like it or not, cybersecurity is a big part of practicing medicine in 2026

What medical practices need to know about risk in 2026
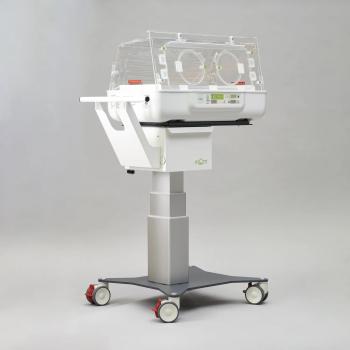
The mOm Essential Incubator wins U.S. regulatory clearance as hospitals seek new ways to expand access to neonatal care, particularly in rural and underserved communities, while reducing the need to separate newborns from their parents.

What you need to know about the biggest trends that will impact health care in 2026

What medical practices need to know about risk in 2026

The outlook for medical device manufacturing in the coming year
Quantis CVP is designed to provide a noninvasive alternative that can be used at the bedside in both adult and pediatric patients.

What medical practices need to know about risk in 2026
The market is expected to hit a compound annual growth rate of 6.6%.

The outlook for medical device manufacturing in 2026
Deal with Robotron Surgical Technologies adds visualization-enabled instruments and AI-focused software roadmap aimed at technology-enabled spine care.

The outlook for medical device manufacturing in the coming year

System is the first cartridge-based CBC system to receive a CLIA Waiver from the FDA, allowing it to be used in settings such as physician offices and urgent care centers without the need for a certified laboratory.

The outlook for medical device manufacturing in the coming year

Deal launches Heartstream as a standalone company and marks the start of ECH’s global platform strategy in the pre-hospital emergency medical technology market.

Expandable interbody fusion implant combines the company’s patient-specific OptiMesh platform with a nano-scale hydroxyapatite surface designed to support bone growth and implant integration

The outlook for medical device manufacturing in the coming year

Integration of NVIDIA’s Blackwell-based IGX Thor platform aims to deliver real-time imaging, robotic control and cloud-enabled intelligence for minimally invasive GI procedures

The outlook for medical device manufacturing in the coming year