
A conversation with Sarah Matt, MD, author of “The Borderless Healthcare Revolution.”
Todd Shryock is managing editor of Medical Economics.

A conversation with Sarah Matt, MD, author of “The Borderless Healthcare Revolution.”

A conversation with Sarah Matt, MD, author of "The Borderless Healthcare Revolution."

A conversation with Sarah Matt, MD, author of “The Borderless Healthcare Revolution.”

Private equity is still looking at medical practices, but they are looking for different things than before
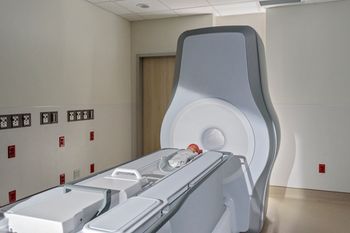
Compact 3T scanner from Eyas Medical Imaging designed for installation inside neonatal intensive care units aims to reduce infant transport risks while delivering high-resolution diagnostic imaging.

Is your practice vulnerable to payment processing fraud and chargebacks?

A conversation with Sarah Matt, MD, author of “The Borderless Healthcare Revolution.”

Is your practice vulnerable to payment processing fraud and chargebacks?
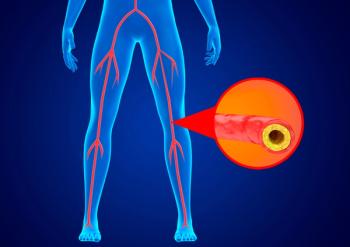
Device aims to improve circulation for patients with peripheral arterial disease at home

A conversation with Sarah Matt, MD, author of “The Borderless Healthcare Revolution.”

Patients are paying more for health care. But what does that mean for your practice?

Study shows low stroke, mortality rates in high-risk patients treated with endovascular arch device

Is your practice vulnerable to payment processing fraud and chargebacks?

A conversation with Sarah Matt, MD, author of “The Borderless Healthcare Revolution.”

Mobile, AI-enabled imaging platform designed to improve workflow and precision in interventional suites

Is your practice vulnerable to payment processing fraud and chargebacks?

A conversation with Sarah Matt, M.D., author of “The Borderless Healthcare Revolution.”

Is your practice vulnerable to payment processing fraud and chargebacks?

Is your practice vulnerable to payment processing fraud and chargebacks?

As physician practices continue to modernize their operations, payments have quietly become one of the most complex—and risky—parts of running a business.

Is your practice vulnerable to payment processing fraud and chargebacks?

Don't let your practice be a victim of - or complicit with - fraudsters

Is your practice vulnerable to payment processing fraud and chargebacks?

The outlook for medical device manufacturing in the coming year

What medical practices need to know about risk in 2026
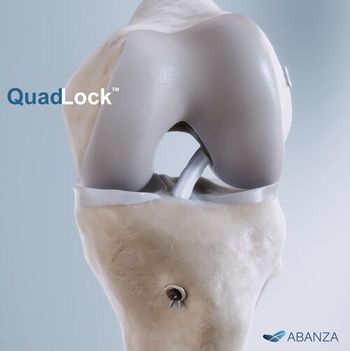
System is intended to securely fixate sutures and tapes while allowing surgeons to fine-tune graft tension and maintain stability across several common ACL graft configurations, including quadriceps tendon, quadrupled semitendinosus/gracilis, and bone–patellar tendon–bone grafts.

Is your practice vulnerable to payment processing fraud and chargebacks?

How many of these possible hazards are at your practice?

What medical practices need to know about risk in 2026
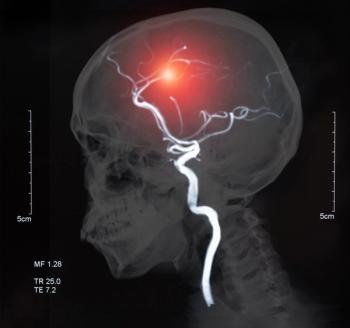
Largest data set to date suggests Hyperfine’s AI-powered, ultra-low-field scanners can identify small ischemic lesions quickly and accurately
Published: August 27th 2020 | Updated:

Published: July 16th 2024 | Updated:
Published: April 25th 2025 | Updated:

Published: August 22nd 2025 | Updated:

Published: October 22nd 2021 | Updated:

Published: September 3rd 2020 | Updated: