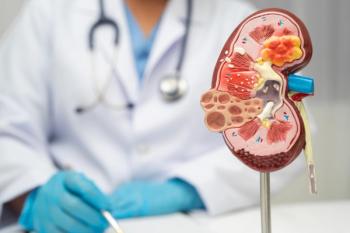
The partnership aims to improve early detection and management of CKD in high-risk and underserved populations by integrating Carna Health’s digital platform with Siemens Healthineers’ global diagnostics infrastructure.
Todd Shryock is managing editor of Medical Economics.

The partnership aims to improve early detection and management of CKD in high-risk and underserved populations by integrating Carna Health’s digital platform with Siemens Healthineers’ global diagnostics infrastructure.

What physicians need to know about AI governance, safety, and responsible use in patient care.
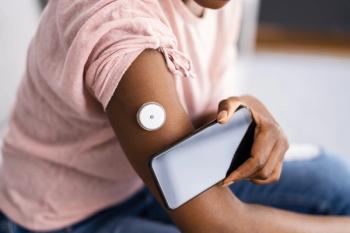
Glucose biosensor combined with artificial intelligence-powered platform provides real-time insights into how food, exercise, stress, and sleep affect the body.

How medical device manufacturing can be brought back to the US

What physicians need to know about AI governance, safety, and responsible use in patient care.

How medical device manufacturing can be brought back to the US

What physicians need to know about AI governance, safety, and responsible use in patient care.

How medical device manufacturing can be brought back to the US

What physicians need to know about AI governance, safety, and responsible use in patient care.

The partnership will combine Vyome’s health care industry expertise and resources with Embryyo’s medical technology capabilities, with the goal of advancing smart medical devices that could reshape disease management and treatment.

How medical device manufacturing can be brought back to the US

What physicians need to know about AI governance, safety, and responsible use in patient care.
Nalu Medical unveils a compact wearable device for chronic pain therapy, enhancing comfort and expanding treatment options for patients with peripheral nerve stimulation.

How medical device manufacturing can be brought back to the US

AI adoption and collaboration trends in the medical device industry

67 health organizations sign letter urging faster access and coverage

What physicians need to know about AI governance, safety, and responsible use in patient care.

FTC challenge could potentially impact heart valve innovation and patient access in the TAVR market.

How medical device manufacturing can be brought back to the US

What physicians need to know about AI governance, safety, and responsible use in patient care.

What physicians need to know about AI governance, safety, and responsible use in patient care.
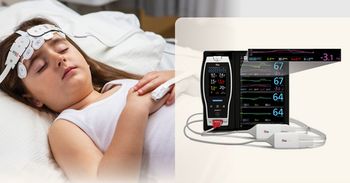
Masimo's O3 Regional Oximetry gains FDA clearance, enhancing tissue oxygen monitoring for improved patient care in critical settings.

How medical device manufacturing can be brought back to the US
Cohesys pioneers drill-free fracture repair with BoneTape, offering a patient-friendly alternative for craniofacial injuries and enhancing recovery experiences.

How medical device manufacturing can be brought back to the US

How medical device manufacturing can be brought back to the US

How medical device manufacturing can be brought back to the US
Philips invests over $150 million in U.S. medtech manufacturing, expanding facilities and creating jobs to enhance AI-driven health care solutions.

How medical device manufacturing can be brought back to the US

The acquisitions give StatLab, a developer and manufacturer of medical diagnostic supplies and equipment, a direct foothold in France, Germany, Italy and the United Kingdom.